Morphic Therapeutic leverages digital chemistry strategy to design a novel small molecule inhibitor of α4β7 integrin
Collaborative enterprise platform and physics-based digital assays empower a team of experts to tackle a challenging target — α4β7 integrin.
Performed rapid in silico design cycles with collaborative platform
Balanced potency, selectivity, and permeability using a structure-based design strategy
Resulted in a development candidate currently in Phase 2b
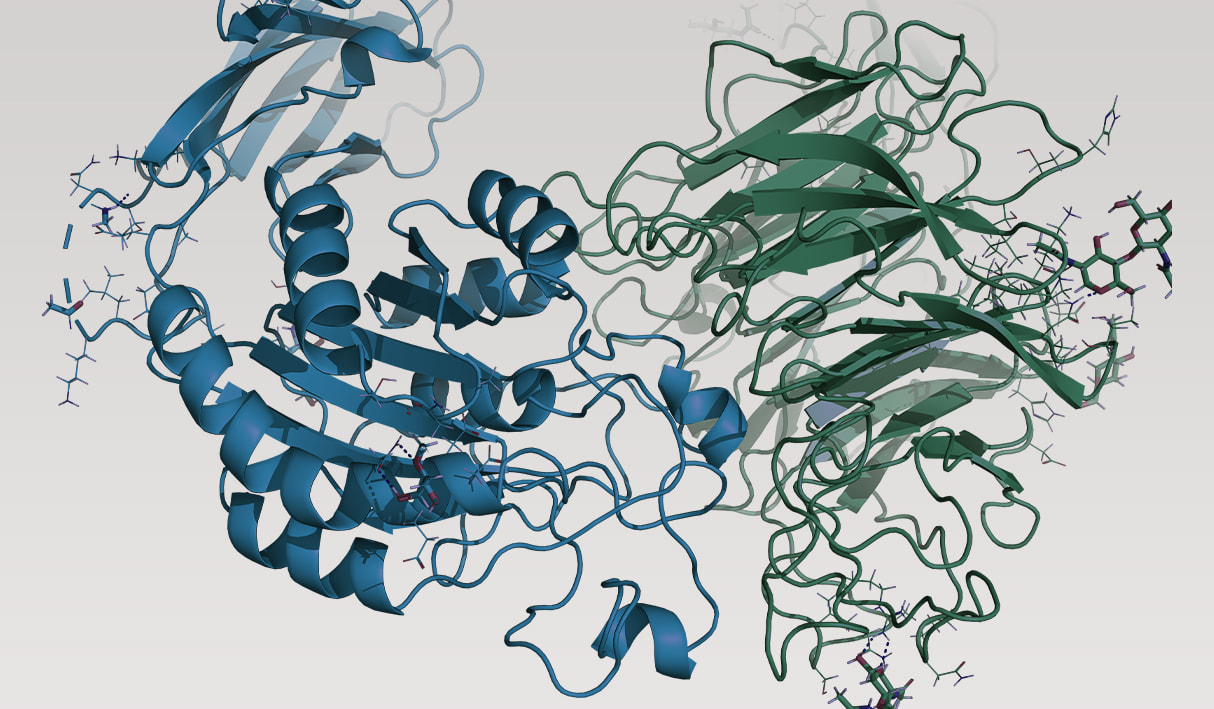
a4B7 integrin
Collaborative program, small molecule
Inflammatory bowel diseases (ulcerative colitis, Crohn’s disease)
Phase 2b clinical trial
“By combining Morphic’s integrin drug hunters with Schrödinger’s in silico molecular design technologies, we were able to successfully deliver a novel small molecule with the key potency, selectivity, and PK properties for this highly validated biological target where others had previously failed.”
Matt Bursavich
VP, Head of Chemical Sciences
Morphic Therapeutic
Design challenge
α4β7 integrin is a well validated target for the treatment of inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis. The approved biologic vedolizumab is an antibody targeting α4β7 and is delivered via injection or infusion, severely limiting accessibility and affordability. An orally bioavailable small molecule therapeutic would represent an enormous step forward in addressing these diseases on a global scale. Despite R&D efforts for over 20 years, the design of potent and selective small molecule inhibitors of α4β7 has been unsuccessful due in large part to the daunting task of overcoming the protein’s structural similarity to other integrin family members. Furthermore, maintaining high potency while balancing optimal physical properties in the design of an oral small molecule inhibiting protein-protein interactions is a formidable challenge.
Morphic Therapeutic, experts in all things integrin, partnered with Schrödinger’s drug discovery team to design an orally bioavailable drug selectively targeting α4β7 integrin to treat inflammatory bowel disease. The goal of the program was to leverage a digital chemistry approach, using the power of physics-based predictive modeling technologies combined with Morphic’s unique integrin biology and chemistry insights, to design a highly selective α4β7 inhibitor with best-in-class properties.
Driving efficient, data-driven design cycles with LiveDesign
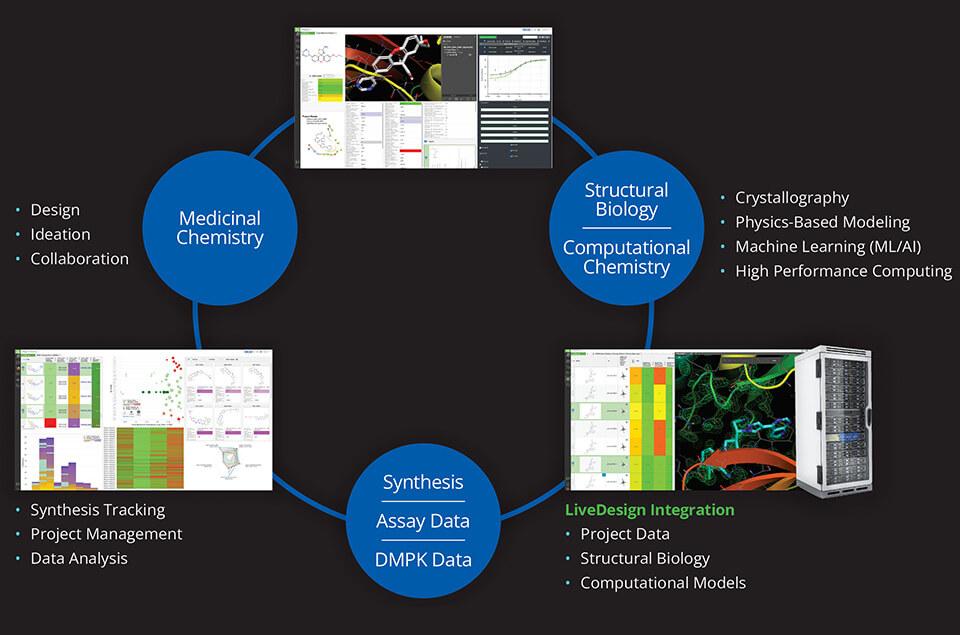
Dispersed teams and remote work environments can pose a significant obstacle to real-time collaboration in a drug discovery program. LiveDesign, Schrödinger’s cloud-based enterprise informatics platform, enables project teams to design compounds, run predictive models, and analyze experimentally-generated data in one place, allowing teams to drive design-maketest- analyze (DMTA) cycles either independently or collaboratively in teams. Centralizing data access and design tools within LiveDesign was imperative to the project team’s ability to tackle their α4β7 integrin inhibitor design challenge (Figure 1).
Using LiveDesign, the Morphic and Schrödinger teams efficiently evaluated and triaged thousands of design ideas much faster than with traditional methods, with far fewer meetings required. Using LiveDesign as the data and predictive modeling platform allowed the teams to utilize a multi-parameter optimization (MPO) strategy driven by advanced data analytics, accurate biophysical modeling of potency and selectivity, and predictive models for pharmacokinetic (PK) properties. Enabled by seamless integration of data, visualization, and predictive modeling, the team rapidly prioritized molecules for synthesis and testing, which resulted in the identification of novel chemical matter that combined the key properties required to overcome all hurdles.
Gaining potency and balancing ADMET properties through rigorous computational assays
Through generation of over 100 proprietary co-crystal structures of inhibitor-bound α4β7 as well as α4β1 — a key off-target — the project team gained significant novel insight into the structure-activity relationships (SAR) of potency and selectivity. However, balancing PK properties with potency remained unsolved. To address this challenge the team combined advanced pKa prediction (a parameter known to correlate with oral bioavailability) using quantum mechanics (Jaguar) with free energy perturbation simulations (FEP+) for accurate potency prediction. The accuracy and utility of FEP+ as a computational assay for the prediction of relative binding energies of molecules has been validated extensively, generating predictions within 1.0 kcal/mol of experimental values on average.1
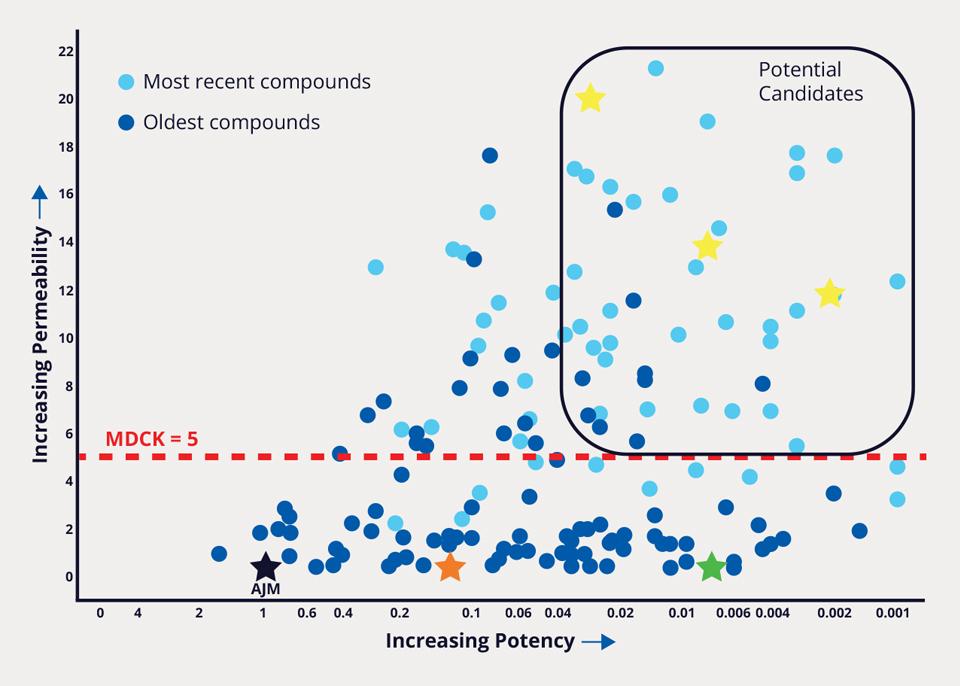
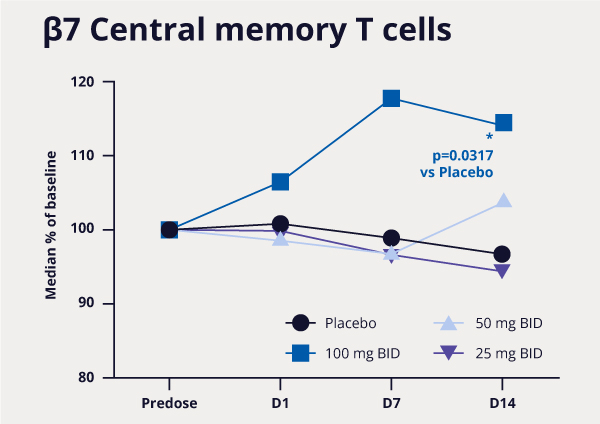
Over 8,500 FEP+ calculations were performed, enabling the team to improve potency 1000-fold, while simultaneously addressing PK liabilities. In parallel, a membrane permeability prediction model was applied to each design as part of the MPO strategy. Compounds that met the computational MPO were then synthesized in the lab. All resulting data obtained for synthesized analogs — both modeled and experimentally derived — were stored and analyzed in LiveDesign. Compounds that were identified to satisfy potency, selectivity, and permeability criteria were aggressively pursued with further characterization (Figure 2). The team’s efforts culminated in the design and delivery of MORF-057, a clinical candidate currently in Phase 2b development for treatment of ulcerative colitis (Figure 3).

Enabling digital technologies to drive discovery programs
FEP+
Digital assay for predicting proteinligand binding across broad chemical space at an accuracy matching experimental methods.
LiveDesign
Collaborative enterprise informatics platform for centralizing access to virtual and wet lab project data and powerful computational predictions.
References
-
Advancing drug discovery through enhanced free energy calculations.
Abel et al. Acc. Chem. Res. 2017, 50, 7, 1625–1632.
-
Accelerating first-in-class and best-in-class programs using a large-scale digital chemistry strategy.
Bursavich et al. Endpoints Webinar.
-
MORF-057, an oral selective α4β7 integrin inhibitor for Inflammatory Bowel Disease, leads to specific target engagement in a single and multiple ascending dose study in healthy subjects.
Ray et al. ECCO 2021.
Software and services to meet your organizational needs
Industry-Leading Software Platform
Deploy digital drug discovery workflows using a comprehensive and user-friendly platform for molecular modeling, design, and collaboration.
Research Enablement Services
Leverage Schrödinger’s team of expert computational scientists to advance your projects through key stages in the drug discovery process.
Scientific and Technical Support
Access expert support, educational materials, and training resources designed for both novice and experienced users.