SGR-1505
Investigators interested in participating in this clinical program, please click below.

Investigators interested in participating in this clinical program, please click below.
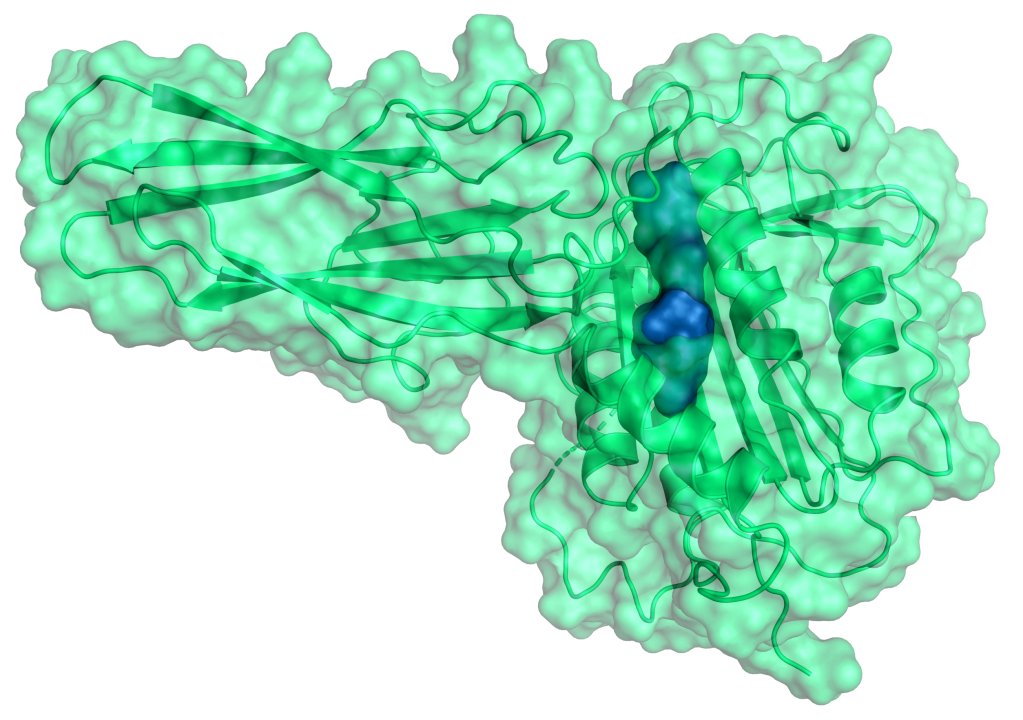
SGR-1505 is an investigational mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) inhibitor.
MALT1 is a key mediator of the NF-κB signaling pathway and the main driver of a subset of B-cell lymphomas. Ongoing, external clinical studies are showing promise of MALT1 as a potential therapeutic target for the treatment of several non-Hodgkin’s B-cell lymphomas, including activated B cell-like diffuse large B cell lymphoma (ABC-DLBCL), mantle cell lymphoma, and chronic lymphocytic leukemia.1,2 MALT1 inhibition also has potential in solid tumors and in autoimmune diseases.
MALT1 is a key mediator of the NF-κB signaling pathway and the main driver of a subset of B-cell lymphomas. Ongoing, external clinical studies are showing promise of MALT1 as a potential therapeutic target for the treatment of several non-Hodgkin’s B-cell lymphomas, including activated B cell-like diffuse large B cell lymphoma (ABC-DLBCL), mantle cell lymphoma, and chronic lymphocytic leukemia.1,2 MALT1 inhibition also has potential in solid tumors and in autoimmune diseases.

We leveraged our physics-based computational platform to rapidly identify novel MALT1 allosteric inhibitors with high potency, high specificity and other important drug-like properties. Multiple tumor models confirmed the potent anti-tumor activity of SGR-1505 as a monotherapy and in combination with standard of care agents for the treatment of B cell malignancies.
SGR-1505 is currently in Phase 1 clinical development for treating patients with relapsed or refractory B-cell neoplasms.
A Phase 1 clinical trial of SGR-1505 is ongoing to evaluate the safety, pharmacokinetics, pharmacodynamics, and preliminary signals of therapeutic activity in patients with relapsed or refractory B-cell lymphomas.
A Phase 1 clinical trial is ongoing to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of SGR-1505 in healthy subjects.
Schrödinger does not currently have an approved access program for our investigational products. We encourage patients to speak with their physicians about treatment options that may be right for them.
Explore resources for patients and physicians.


WO2022184716 Combination Therapy using MALT1 Inhibitor and BTK Inhibitor.
WO2022185097 Method of treating a condition using a therapeutically effective dose of the MALT1 inhibitor JNJ-67856633.