SEP 12, 2024
Accelerated in silico discovery of SGR-1505: A potent MALT1 allosteric inhibitor for the treatment of mature B-cell malignancies
Abstract:
MALT1 (Mucosa-associated lymphoid tissue lymphoma translocation protein 1) is a component of the MALT1-BCL10-CARD11 complex downstream from the Bruton Tyrosine Kinase (BTK) on the B-cell receptor signaling pathway. MALT1 is a key mediator of nuclear factor kappa B (NF-κB) signaling, which is the main driver of a subset of B-cell lymphomas. MALT1 is considered a potential therapeutic target for several subtypes of non-Hodgkin’s B-cell lymphomas and chronic lymphocytic leukemia (CLL), including tumors with acquired BTK inhibitor (BTKi) resistance. Constitutive activation of the NF-κB is a molecular hallmark of activated B cell-like diffuse large B cell lymphoma (ABC-DLBCL), and MALT1 may have utility as a treatment option for ABC-DLBCL. Furthermore, a third-party MALT1 inhibitor recently showed strong anti-tumor activity in mature B cell malignancies from Phase 1 studies.
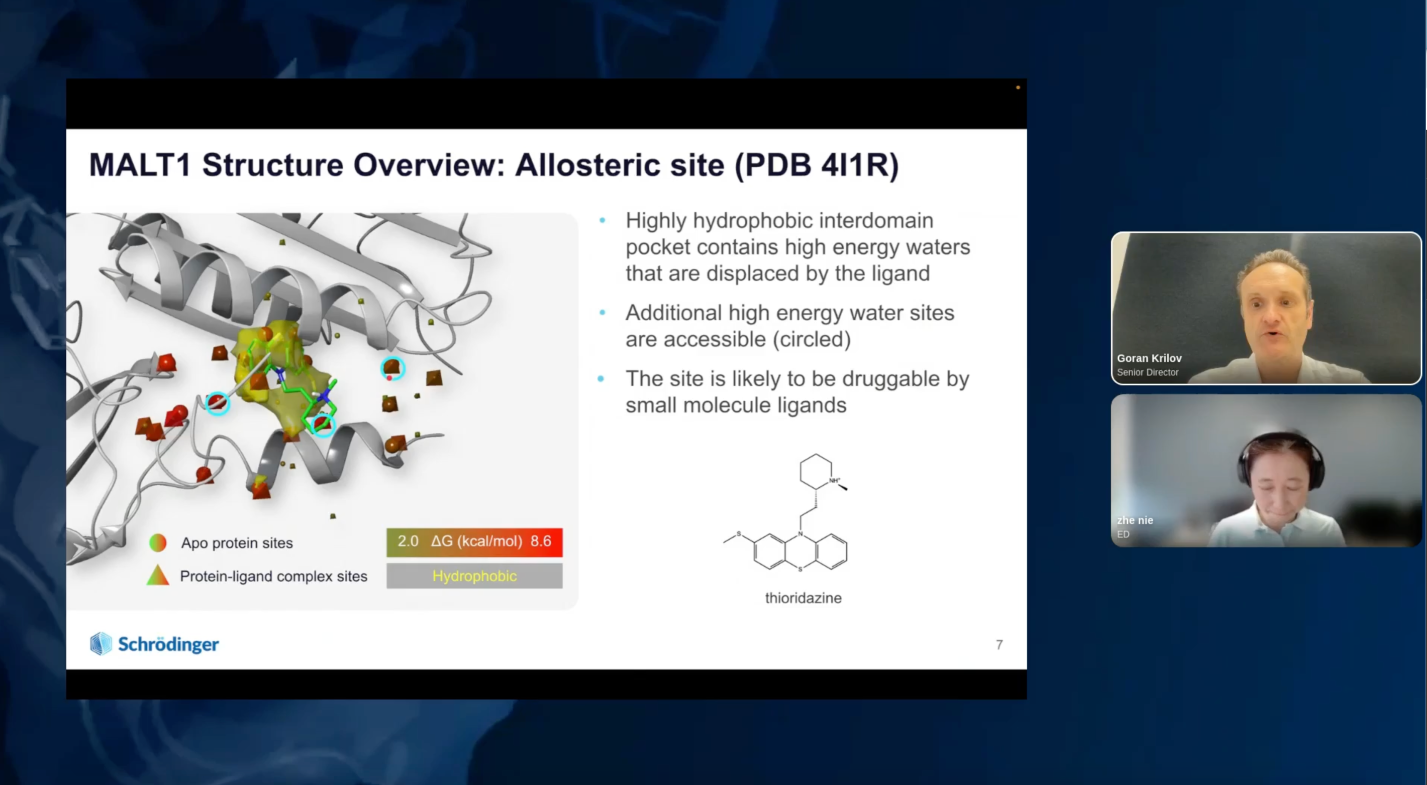
By applying advanced physics-based modeling techniques, including combining free energy calculations with machine learning methods and chemistry-aware compound enumeration workflow, the Schrödinger team explored extensive sets of de novo design ideas to quickly identify a novel hit series with an in vivo tool molecule to establish an in vivo PD and efficacy mouse model early on in the project. Multi-parameter optimization (MPO) allowed efficient prioritization of molecules with good potency and drug-like properties during lead optimization. This led to the discovery of a highly potent MALT1 inhibitor, SGR-1505, with a well-balanced property profile in under a year, with only 78 compounds synthesized in the lead series and 129 compounds overall. SGR-1505 is a potent and orally available allosteric MALT1 inhibitor. It demonstrated strong anti-tumor activity alone and in combination with BTK inhibitors in multiple in vivo B-cell lymphoma xenograft models. Currently, a Phase 1 clinical trial with SGR-1505 in patients with mature B-cell neoplasms is ongoing (NCT05544019).
Webinar Highlights:
- Discover how free energy calculations, amplified by machine learning methods, led to the discovery of a highly potent MALT1 inhibitor, SGR-1505
- Learn how the team used an MPO scoring function consisting of FEP+-based predictions of affinity and solubility, physics-based predictions of permeability, and predictions of lipophilicity to optimize compounds
- Ask questions to gain further insight from the speakers to apply to your work
Our Speakers

Goran Krilov
Senior Director, Schrödinger
Dr. Goran Krilov is a senior Director of Computational Chemistry at Schrödinger’s Therapeutic Group. For the past twenty five years, his work has focused on developing and applying cutting-edge computational chemistry techniques to problems in biophysics and drug discovery. He has led the modeling efforts on a number of internal projects as well as external collaborations in oncology and neurogenerative diseases, resulting in two clinical candidates currently undergoing Phase I trials. Prior to joining Schrödinger, Dr. Krilov has worked in both industry and academia, including IBM, Boston College snd Strand Life Sciences.

Zhe Nie
Executive Director, Schrödinger
Dr. Zhe Nie is the Executive Director of Medicinal Chemistry at Schrödinger’s Therapeutic Group. She has been leading multiple wholly owned and partnered drug discovery programs at Schrödinger. Most recently, she led Schrödinger’s MALT1 discovery project team, successfully developed the small molecule drug SGR-1505 (Schrödinger’s first internal clinical asset currently in Ph1) applying Schrödinger’s computational platform. It took less than two years from the start of the project to the selection of the clinical candidate. She also led the DLK collaboration project with Takeda Pharmaceuticals which discovered a potent, selective, and brain-penetrate DLK inhibitor as a promising preclinical candidate for the treatment of neurodegenerative diseases using Schrödinger’s computational platform. She has extensive experiences in applying advanced computational tools to assist in the design of small molecule drug candidates. She previously worked at Takeda, Celgene and Quanticel Pharmaceuticals (acquired by Celgene), led and contributed to advancing multiple small molecule drugs to the clinics including TAK-960, TAK-659 and CC-90011.