SGR-3515
Investigators interested in participating in this clinical program, please click below.

Investigators interested in participating in this clinical program, please click below.
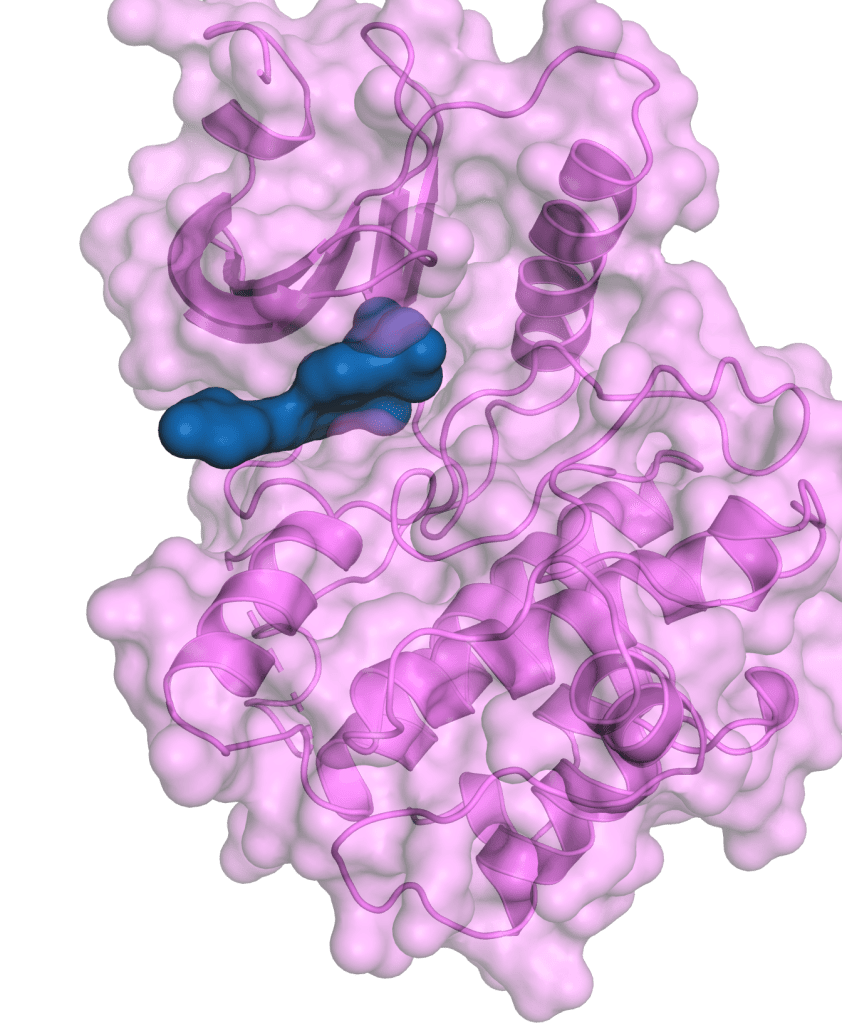
SGR-3515 is a Wee1/Myt1 inhibitor.
Wee1 and Myt1 (membrane associated tyrosine/threonine 1) kinases regulate the cell cycle and DNA damage response through phosphorylation and inactivation of cyclin dependent kinase 1 (CDK1), allowing cells to repair DNA damage before entering mitosis. Concurrent loss of function of Wee1 and Myt1 confers selective vulnerability in cancer cells, a mechanism referred to as synthetic lethality. Compounds inhibiting Wee1 and Myt1 have shown clinical benefit in gynecological and other cancers.

We leveraged our computational platform to identify multiple highly selective and structurally distinct Wee1 inhibitors with optimized physicochemical properties. Preclinical models confirmed strong pharmacodynamic responses and anti-tumor activity of SGR-3515 as a monotherapy and as part of combination therapy with other agents. SGR-3515 is a Wee1/Myt1 dual inhibitor with a differentiated biochemical, biophysical and functional profile, with sustained inhibition of Wee1 and Myt1 in tumor tissue. Myt1 co-inhibition enhances activity and leverages synthetic lethality, with potential to reduce tumor resistance. The profile of SGR-3515 enables dosing schedules optimized to maintain anti-tumor activity and limit hematological toxicity.
SGR-3515 is currently in clinical development in patients with advanced solid tumors.
Patient trial
A Phase 1 clinical trial of SGR-3515 is ongoing to evaluate the safety and tolerability and to determine the maximum tolerated dose/recommended dose in patients with advanced solid tumors.
Learn more at: CLINICALTRIALS.GOV
Explore resources for patients and physicians.
