Advancing the design and optimization of drug formulations with combined computational and experimental approaches
Scientists from AbbVie and Schrödinger collaborated to systematically investigate amorphous solid dispersion (ASD) dissolution behaviors by combining thermodynamic modeling, molecular simulation, and experimental research.
Summary
Investigated ASD/water interfacial gel layer behavior with thermodynamic modeling and confirmed this behavior by microscopic erosion time test (METT) experiments
Acquired additional insights of ASD dissolution at the molecular level, which validated the results from thermodynamic modeling and METT experiments, and inspired the design of drug formulations
Gained a deeper understanding of the drug load-dependent release mechanism and the occurrence of loss of release of a complex ASD formulation that is comparable to commercially available formulations
Challenges
During the dissolution of amorphous solid dispersion (ASD) formulations, the drug load (DL) often impacts the release mechanism and the occurrence of loss of release (LoR). The effect has been experimentally observed and discussed in the literature. However, the underpinning principles and mechanisms are less investigated and reported, since they require a better understanding of the thermodynamics and molecular interactions of ASD dissolution. A combined approach leveraging molecular-level simulations and thermodynamic modeling is uncommon in the literature, but can provide deeper insights into the complex phase behavior of ASDs during dissolution.
Approach
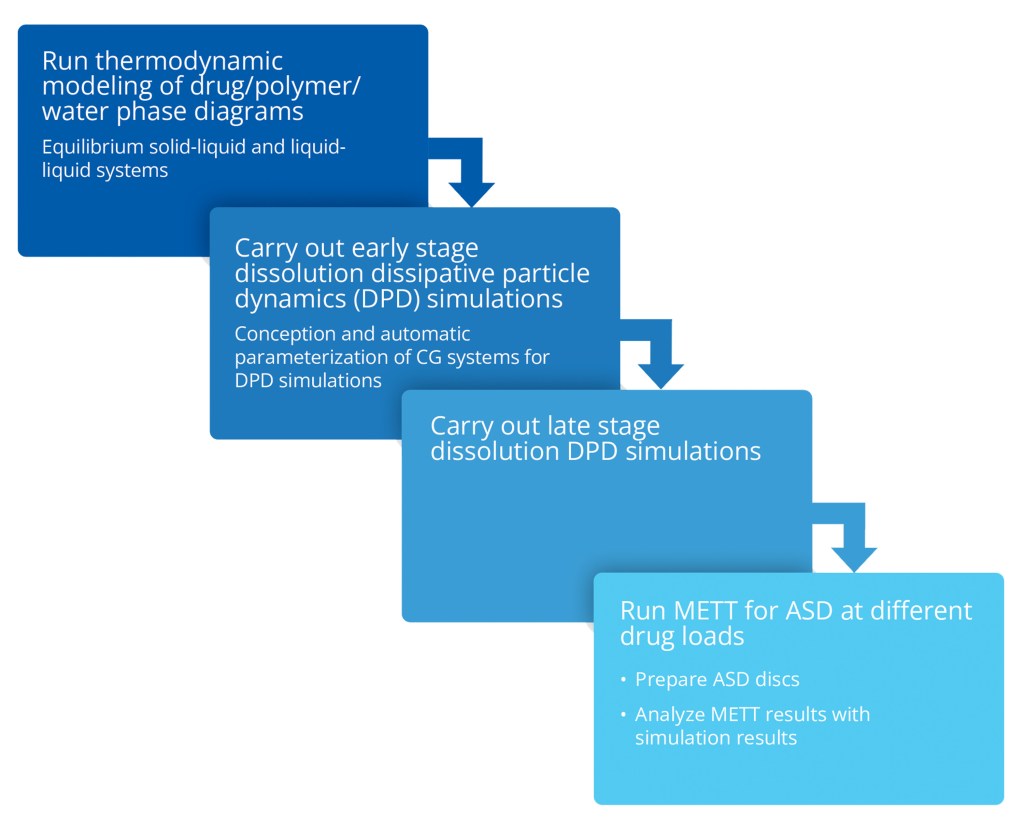
Continuing their collaboration on ASD dissolution R&D,1-2 scientists from AbbVie and Schrödinger extended this research to use a combined experimental-computational approach to investigate the drug load-dependent release mechanism and the occurrence of loss of release in ASDs containing the drug ritonavir and the polymer copovidone (PVPVA64). All molecular simulations were performed using Schrödinger’s Materials Science platform (MS Maestro) and Desmond for molecular dynamics (MD) and coarsegrained (CG) simulations.
Results and outlook
This study3 demonstrates that the complementary information gained from thermodynamic modeling, molecular simulation, and experiment provides a valuable and more complete understanding of the release mechanism of ritonavir/PVPVA64-based ASDs.
- At low to moderate DL (5–15 wt%), PVPVA64 formed the major phase (polymer-rich phase), while ritonavir formed the minor phase (drug-rich phase) and the drug release is dictated by its solubilization. At high DL (20–40 wt%), self- and drug-polymer interactions play a larger role.
- For high DL (40 wt%) ASDs, at the early stages of hydration, the drug molecules distinctively aggregate into drug-rich phase clusters near the surface of the ASD, delaying erosion and drug release considerably.
- In the late dissolution simulations, the interactions between the ritonavir molecules and the polymer’s monomers, as well as with the water molecules, were tracked to better understand the mutual association between drug, polymer, and solvent.
- Interaction analysis for late stage simulations focusing on the drug-rich clusters revealed how dehydration and stabilization of these clusters increases with high DLs.
This approach can support the identification of maximal DL as a possible starting point for further ASD formulation design and development. The predictive power of the presented models provides scientists with powerful tools to overcome challenges in optimizing drug formulations.
Methodology implemented for molecular simulation component of the investigation
Automatic parameterization of coarsegrained (DPD) systems
Seamless simulation studies of complex formulations
Description of complex dissolution/ organization patterns
References
-
Case study
Advancing the design and optimization of drug formulations with coarse-grained molecular simulations
-
Molecular-Level Examination of Amorphous Solid Dispersion Dissolution
Mohammad Atif Faiz Afzal, Kristin Lehmkemper, Ekaterina Sobich, Thomas F. Hughes, David J. Giesen, Teng Zhang, Caroline M. Krauter, Paul Winget, Matthias Degenhardt, Samuel O. Kyeremateng, Andrea R. Browning, and John C. Shelley Mol. Pharmaceutics 2021, 18, 11, 3999-4014
-
Predicting the Release Mechanism of Amorphous Solid Dispersions: A Combination of Thermodynamic Modeling and In Silico Molecular Simulation
Stefanie Walter, Paulo G. M. Mileo, Mohammad Atif Faiz Afzal, Samuel O. Kyeremateng, Matthias Degenhardt, Andrea R. Browning and John C. Shelley Pharmaceutics 2024, 16(10), 1292